
EPISKIN and EPISKIN Academy are attending the WCD from June 10-15, 2019, in Milan, Italy.
Episkin presents 2 new posters and will do an oral presentation:
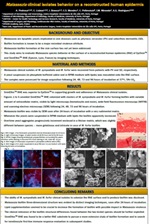
- Malassezia clinical isolates behavior on a reconstructed human epidermis
AF Pedrosa1,2,3*, C Lisboa1,2,3, J Branco1, CS Mendes4, C Pellevoisin5, IM Miranda1,3, AG Rodrigues1,3
1 Division of Microbiology, Department of Pathology, Faculty of Medicine, University of Porto, Portugal
2 Department of Dermatology and Venereology, Centro Hospitalar São João EPE, Porto, Portugal
3 CINTESIS - Center for Health Technology and Services Research, Faculty of Medicine of the University of Porto, Portugal
4 Department of Surgery Physiology and Cardiovascular R&D Center, Faculty of Medicine, University of Porto, Portugal
5 EPISKIN Academy, Lyon, France
6 Burn Unit, Department of Plastic and Reconstructive Surgery, Centro Hospitalar São João EPE, Porto, Portugal
Introduction: Malassezia yeasts are implicated in skin diseases such as pityriasis versicolor (PV) and seborrheic dermatitis (SD). Biofilm formation represents a major virulence attribute for microorganisms. Malassezia biofilm formation at the skin surface has not yet been investigated.
Objective: To evaluate how Malassezia clinical isolates behavior on the surface of a reconstructed human epidermis (RHE) of EpiSkinTM and SkinEthicTM RHE (Episkin, Lyon, France).
Material and Methods: Malassezia clinical isolates were recovered from volunteers with PV, SD and participants free of lesions at an University Hospital from 2013 to 2016. The prospective enrollment of volunteers was initiated after approval by the Ethics Committee of Health. Two clinical strains of M. furfur (from SD) and M. sympodialis (from PV) were added to the RHE. The tissues were processed for image acquisition.
Results: There was colonization of the RHE with aggregates of Malassezia visible by histology, confocal microscopy and scanning electron microscopy (SEM) at 24 hours. The latter showed the yeasts adherence to the RHE surface and attachment to each other embedded within a slimy extracellular matrix which confirms the biofilm formation at the RHE surface for these 2 Malassezia species. At 48 hours it was apparent a slightly increase in the number of microorganisms with expansion of these communities of surface-associated cells enclosed in a thicker matrix, especially for M. sympodialis. SkinEthicTM RHE was superior to EpiSkinTM in supporting the growth and adherence of Malassezia clinical isolates.
Conclusions: The ability of M. furfur and M. sympodialis strains to colonize the RHE surface forming biofilm was hereby demonstrated; its three-dimensional structure was evident by confocal microscopy and SEM. As a preliminary research unveiling Malassezia interaction on the surface of RHE, we found SkinEthicTM RHE as the best RHE to follow a more extensive approach with biofilm formation and host response evaluation.
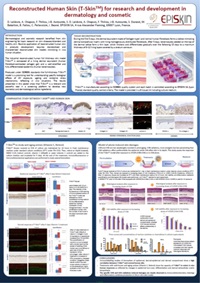
- Reconstructed Human Skin (T-SkinTM) for research and development in dermatology and cosmetic
D. Lelièvre, A. Chapuis, F. Thillou, J-B. Autourde, S. Durand, M. Bataillon, B. Fallou, C. Pellevoisin, I. Besné.
EPISKIN SA, 4 rue Alexander Fleming, 69007 Lyon, France.
Background:
Dermatological and cosmetic research benefited from skin engineering for basic research on skin diseases/disorders and healthy skin. Routine application of reconstructed human skin in products development requires standardized and characterized reconstructed skin models mimicking in vivo human situation.
Objective: Studying T-Skin™ a new industrial reconstructed human skin model with a self-renewing epidermis and a living dermis:
• Comparative study between T-SkinTM and human skin
• Functional response of T-SkinTM to anti-aging actives
• Model of photo-induced skin damages
Materials and Methods:
-Normal human skin (NHS) from plastic surgery and T‐Skin™are compared by histology and immunohistochemistry (transglutaminase-1, keratin-10, Ki67, collagen IV, fibrillin and pro‐collagen-I).
-T-SkinTM models are exposed 5 days to Retinol (10μM) or Vitamin C (200μM) then dermal-epidermal junction, dermal biomarkers (Laminin‐5, Procollagen‐I, Collagen‐IV and VII) and epidermal biomarkers (Ki67, transglutaminase-1 and cytokeratin 10) are evaluated.
-T-Skin™ models are exposed to increasing doses of UVA or UVSSR (UVA+B) from 0 to 55J/cm2. 24h after, histology and cytokines quantification (IL-1a, IL-1ra, IL-6, IL-8, GM-CSF,TNF-a) are performed.
Results:
- Comparative study between T-Skin™ and NHS shows similitude in term of tissue organization and cell differentiation/functionalization at epidermal, dermal-epidermal and dermal compartments.
- Functional response of T-SkinTM after exposure to reference anti-aging compounds (Vitamin C, Retinol) mimics in vivo human responses in term of epidermal turn-over, differentiation and dermal extracellular matrix biosynthesis.
- In the model of photo-induced skin damages, T-Skin™ expresses specific UVA versus UVB modification at epidermal and dermal levels in accordance with known in vivo UV effects.
Conclusions:
T-SKINTM models in vitro some of in vivo NHS properties both in terms of organization and, in terms of response to different stimuli. This makes it a pertinent in vitro screening tool for early and ongoing efficacy assessment during product development.
- Reconstructed human epidermis models with Langerhans cells (SkinEthic RHE-LC)
F. Sahuc1, V. Dioszeghy2, D. Benas1, M. Ligouis2, L. Mondoulet2, C. Pellevoisin1, JM Ovigne1
1- EPISKIN SA, 4 rue Alexander Fleming, 69007 Lyon, France. 2- DBV Technologies, Montrouge, France
Background:
Langerhans cells are a bridge between skin innate and adaptive immunity and are key players in a wide range of immune-mediated skin disorders. Availability of reconstructed human epidermis containing Langerhans cells opens new possibilities for research and development in dermatology and cosmetics.
Objective: Characterization and application in dermatology research of SkinEthicTM RHE-LC, a new industrial reconstructed human epidermis containing functional Langerhans cells.
Materials and Methods:
SkinEthicTM RHE-LC (EPISKIN SA) model is reconstructed on a polycarbonate filter membrane with human primary keratinocytes and human CD34+ progenitor cells. This model is characterized by histology and immunostaining (CD1a, CD207) studies. Functional response of the resident Langerhans cells is assessed by transcriptomic studies (CCR7 et CD86) after topical or systemic exposure of the model to chemical sensitizers (PPD, DNCB).
The relevance of the model to study allergen uptake and activation of the Langerhans cells have been performed by DBV Technologies using FACS analysis of exposed model to OVA-AF488, oxazolone or PBS. OVA-AF488 is applied either directly or with Viaskin® (DBV Technologies) patches used in vivo for epicutaneous immunotherapy (EPIT).
Results:
Immunolabelling of histological slices with CD1a and CD207 antibodies show regular repartition of Langerhans cells in the basal and suprabasal layers of the model. 24h hours after exposure to sensitizers CCR7 et CD86 expression are upregulated reflecting Langerhans cells activation. In the antigen capture challenge, 60% and 90% of LC are positive to OVA for the groups treated with OVA-AF488 and the Viaskin®-OVA-AF488, respectively. The expression of HLA, CD80 and PDL2 was not modified by the treatments in comparison to the inflammatory control oxazolone.
Conclusions:
These results show that SkinEthicTM RHE-LC is a functional model of human skin able to mimic some mechanisms of human exposure to skin sensitizers. Moreover, SkinEthicTM RHE-LC is useful for mechanistic investigation on antigen delivery in human.