 |
Depigmentation
|
MODELS
PROTOCOL PRINCIPLE
The purpose of this study is to assess in vitro the depigmentation activity of the test product after daily topical application on the SkinEthicTM RHPE model for 6 days.
Fivefold reconstructed human tanned epidermal tissues (phototype VI), are dosed with the test product for 6 days. After the treatment, all tissues are scored visually. Additionally, one of the 5 tissues is assessed for histology (H&E staining) and one is used for tissue viability assessment. The remaining 3 tissues are used for melanin quantification.
DETAILED ASSAY PROCEDURE
Test method
1 µl of the test product, and 1 µl of positive control equivalent of Melanex DUO ( Hydroquinone) are deposited daily onto the surface of the stratum corneum of fivefold SkinEthicTM RHPE, (size 0.5 cm²). Dosing is performed the first 4 days. All cultures (including fivefold untreated tissues) are consequently incubated at 37°C, 5% CO². Analysis (visual assesment, histology, MTT and melanin extraction) are performed at the end of the treatment period.
NB: this protocol is used for a cream or a gel formulated product; if the test product is a liquid, the protocol can be changed; it is also possible to test a product by systemic application, in the culture medium.
Evaluation of cell viability (MTT test)
For each test condition, duplicate cultures are placed on 300 µl of 0,5 mg/ml MTT and incubated 3 hours at 37° C 5% CO². Extraction is performed in 1.5 ml of isopropanol at room temperature, for a minimum of 2 hours. Optical density is measured at 570 nm. Results are expressed as percentage of viability compared to negative control: % Viability = [OD (570nm) test compound / OD (570nm ) negative control)] x 100.
Histology
For each test condition, one tissue is fixed in a balanced 10% formalin solution and embedded in paraffin. Four micron vertical sections are stained with hematoxylin/eosin and photographed under a microscope.
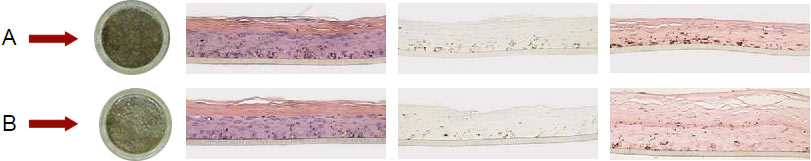
Repeated applications of the skin care cream decrease the tanning degree (B) as compared to the untreated control (A). By Fontana Masson staining, we note a correlation between reduced pigmentation and lower melanin content present in the melanocytes.
Visual assessment
At the end of the test period, all tissues (untreated, positive control and test material treated) are photographed using a digital camera for visual assesment of depigmentation.
Melanin extraction assay
At the end of the test period, the 3 tissues are removed from the insert by cutting out the filter with a sharp scalpel. Each filter is plunged into 360 µl of Solvable (Perkin Elmer) and heated at 100 degrees Celsius for 45 minutes. The optical density are measured on 80 µl extract at 490 nm using synthetic melanin as reference. Results are expressed as OD and as mg/ml melanin.
REFERENCES
Chemical characterisation of hair melanins in various coat-color mutants of mice. Ozeki, H., Ito S., Wakamatsu K. and Hirobe T., Journal of Investigative Dermatology, 105, 3, 361-366, 1995).
Human pigmented epidermis reconstructed in chemically defined medium used for evaluation of modulation of pigmentation. F. Sahuc, B. De Wever & M. Rosdy. Presented at the 12th Meeting of the European Society of Pigmented Cell Research, Paris, Sept. 2004.