Based on easy-to-use and validated protocols, EPISKINTM tissue models allow industrial corporations to screen and measure with high precision, the irritation, penetration, metabolism, or efficacy of large number of formulations or actives, to eliminate those that are unsuitable, so that the final (in vivo or clinical) tests can be carried out on a selected few.
MULTIPLE END-POINT TESTING APPROACH
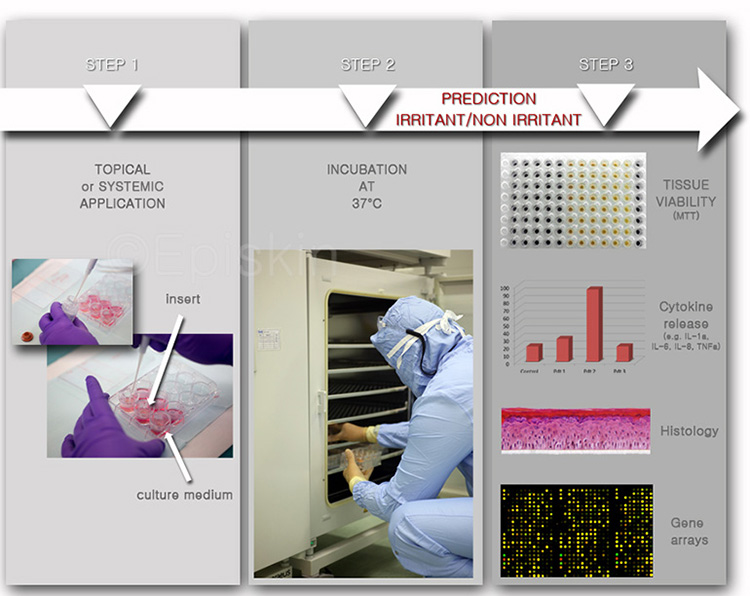
A small amount (2-10 mg/cm²) of test product (and controls) is deposited onto the surface of the tissues and spread with a small paintbrush. The cultures are incubated at 37°C for a specific time (from 10 minutes up to 6 days), after which they are analyzed for tissue histology, viability and the release of inflammatory mediators or cytokines (multiple end-point analysis).
MULTIPLE SAFETY SCREENING

EFFICACY TESTS: FOCUS ON THE MIGRATION TEST
The goal of the migration test is to observe the capacity of actives to improve the skin regeneration through a wound healing like process based on the T-Skin® technology.
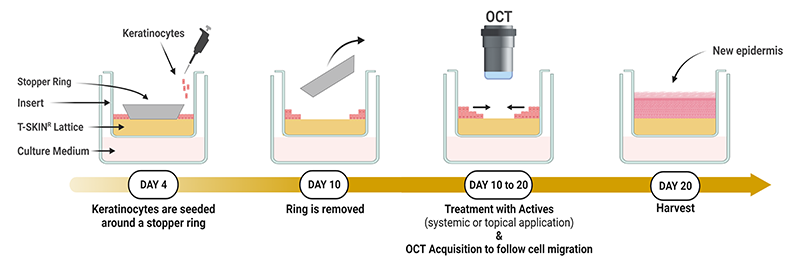
The test is described on the poster and video below.
Migration-WCD 2023
Click on picture above to launch video